

The effect of temperature on the persistence of SARS-CoV-2 on common surfaces
Shane Riddell, Sarah Goldie, Andrew Hill, Debbie Eagles & Trevor W. Drew
Virology Journal 17, Article number: 145 (2020) – Published 07 October 2020.

Abstract
Background
The rate at which COVID-19 spreads throughout the globe has been alarming. While the role of fomite transmission is not yet fully understood, precise data on the environmental stability of SARS-CoV-2 is required to determine the risks of fomite transmission from contaminated surfaces.
Methods
This study measured the survival rates of infectious SARS-CoV-2, suspended in a standard ASTM E2197 matrix, on several common surface types. All experiments were carried out in the dark, to negate any effects of UV light. Inoculated surfaces were incubated at 20 °C, 30 °C and 40 °C and sampled at various time points.
Results
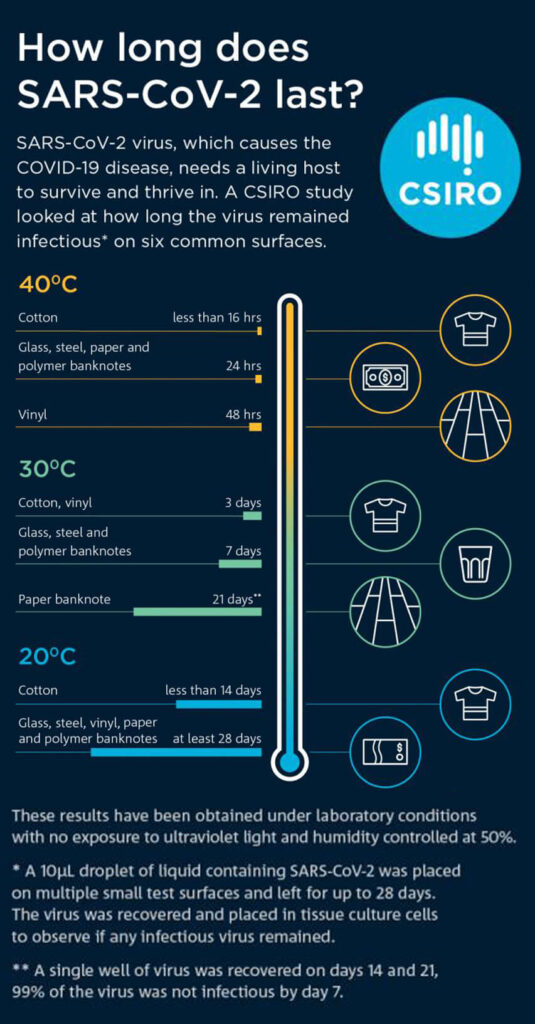
Survival rates of SARS-CoV-2 were determined at different temperatures and D-values, Z-values and half-life were calculated. We obtained half lives of between 1.7 and 2.7 days at 20 °C, reducing to a few hours when temperature was elevated to 40 °C. With initial viral loads broadly equivalent to the highest titres excreted by infectious patients, viable virus was isolated for up to 28 days at 20 °C from common surfaces such as glass, stainless steel and both paper and polymer banknotes. Conversely, infectious virus survived less than 24 h at 40 °C on some surfaces.
Conclusion
Background
Fomite transmission has previously been shown to be a highly efficient procedure, with transmission efficiencies of 33% for both fomite to hand and fingertip to mouth transfer for bacteria and phages [10]. With the high efficiency of fomite transfer, the persistence of SARS-CoV-2 on environmental surfaces is therefore a critical factor when considering the potential for fomite transmission for this virus. Currently, there are conflicting reports on the survivability of SARS-CoV-2, with data ranging from 3 to 14 days at room temperature for a single surface type, stainless steel [5, 11]. This study aims to provide environmental stability data for SARS-CoV-2 under controlled temperature and humidity conditions for a range of common surfaces.
Methods
Virus isolate
Surfaces
Surface inoculation and sampling
Statistical analysis
Results
At 30 °C, infectious virus was recoverable for 7 days from stainless steel, polymer notes and glass, and 3 days for vinyl and cotton cloth. For paper notes, infectious virus was detected for 21 days, although there was less than 1 log of virus recovered for both 14 day and 21 day time points. The D values for surfaces at 30 °C ranged from 1.4 days for vinyl to 4.9 days for paper notes (Table 1).
At 40 °C, virus recovery was significantly reduced compared to both 20 °C and 30 °C experiments. Infectious SARS-CoV-2 was not recovered past 24 h for cotton cloth and 48 h for all remaining surfaces tested. Greater than 4-log reduction (99.99% reduction from starting titre) was observed in less than 24 h at 40 °C on all surfaces. The D values for surfaces at 40 °C have been converted to hours as they were all less than 1 day, values ranged from 5 h for polymer notes to 10.5 h for vinyl (Table 1).
For each temperature and substrate material, the mean titre from three replicates of recovered virus was plotted against time, with standard deviations included. Linear regression was used to calculate a line of best fit. Plots showing virus survival on each substrate at the three temperatures investigated are shown in Fig. 1. Plots presenting this data grouping all substrates at each of the three temperatures are given in Fig. 2. Calculated D-value, Half Life and Z-value are presented in Table 1.
Discussion
While the primary spread of SARS-CoV-2 appears to be via aerosols and respiratory droplets, fomites may also be an important contributor to the transmission of the virus. Fomite transmission has been demonstrated as an important factor in the spread of other coronaviruses such as porcine epidemic diarrhoea virus [16], as well as being suspected for Middle East Respiratory Syndrome coronavirus [17], human coronavirus 229E and OC43 [18] and SARS-CoV-2 [7].
This study utilised a virus concentration of 4.97 × 107/mL diluted into a standard solution which mimics body fluid composition (final concentration of 3.38 × 105/10 µL inoculum), which equates to a cycle threshold (CT) value of 14.2, 14.0 and 14.8 for N gene, E gene and RdRp gene real-time RT-PCR, respectively (unpublished data). Previous studies have shown some patients with high viral loads have recorded CT values of between 13 and 15 [19,20,21]. van Doremalen et al. [5] described their test material (105 TCID50/mL) as having a CT of 20–22, which compared similarly to CTs reported from clinical patients [5, 22]. While the titre of virus utilised in this study is high it represents a plausible amount of virus that may be deposited on a surface.
The present study has demonstrated that in controlled conditions, SARS-CoV-2 at a starting viral load and in a fluid matrix equivalent to that typically excreted by infected patients, remains viable for at least 28 days when dried onto non-porous surfaces at 20 °C and 50% relative humidity. Research on the original SARS virus also showed recovery of infectious virus when dried on plastic for up to 28 days at room temperature and 40–50% RH [23]. Recent data published on SARS-CoV-2 survivability on hospital PPE observed viable virus up to 21 days post-inoculation on both plastic and N95 mask material when held at room temperature [11], correlating with the data presented in this study. The persistence of SARS-CoV-2 on surfaces presented here and from Kasloff et al. [11] demonstrate significantly longer time points than previously published data for SARS-CoV-2 [5, 24]. These earlier studies reported recovery of infectious SARS-CoV-2 up to 3 days post-inoculation and 4 days on non-porous surfaces, respectively. The titre of virus used in this study is at least 2 logs higher than used in the paper by van Doremalen et al. [5], which may account for the longer survivability. Work by Lai et al. has shown that the stability of the SARS virus was enhanced with higher concentrations [25]. Temperature and humidity are both critical factors in viral survivability with an increase in either being detrimental to virus survival [23, 26, 27]. Survivability on stainless steel coupons for transmissible gastroenteritis virus and murine hepatitis virus (both coronaviruses) was reduced with higher humidity’s and temperature [28] and survivability of Middle East Respiratory Syndrome coronavirus also followed a similar pattern [29]. The higher humidity of ~ 65% RH used by Chin et al. [24] may explain the shorter persistence of virus when compared to the data presented here.
SARS-CoV-2 has been shown to be rapidly inactivated under simulated sunlight [30, 31]. To remove any potential decay by light sources, inoculated coupons were held in the dark for the duration of the experiment.
Decimal reduction (D value; the time taken to reduce the titre by 1 log) for SARS-CoV-2 at 20 °C and 50%RH ranged from 5.57 to 9.13 days (average 6.82) for all surfaces tested. This data is significantly longer than modelling predictions performed by Guillier et al. [32]. The data presented here was performed under controlled conditions with fixed temperatures, relative humidity, suspension matrix and in the absence of light, which may explain the enhanced survivability observed in this study. The generation of Z values at different temperatures also allows for the extrapolation of D values for each surface at other temperatures. The Z value represents the temperature change required to alter the D value by 1 log. For stainless steel, the D value was determined to be 6.48 days at 20 °C, and the Z value of 13.62 °C, therefore if the temperature was to drop by 13.62 °C from 20 °C (i.e. to 6.38 °C), then the D value would increase from 6.48 days to over 64 days. This data could therefore provide a reasonable explanation for the outbreaks of COVID-19 surrounding meat processing and cold storage facilities. The data also supports the findings of a recent publication on survival of SARS-CoV-2 on fresh and frozen food [33].
Stainless steel is a common surface for the study of viral stability and has been used to study the persistence of a number of viruses such as the Ebola virus, hepatitis virus, Influenza A and Coronaviruses [28, 34,35,36,37]. This study demonstrates that SARS-CoV-2 is extremely stable on stainless steel surfaces at room temperature (> 28 days at 20 °C/50%RH) however, is less stable at elevated temperatures (7 days at 30 °C and < 48 h at 40 °C). Recovery of the infectious virus on stainless steel has been observed for murine hepatitis virus and transmissible gastroenteritis virus for up to 28 days albeit at a lower humidity 20%RH [28]. Interestingly, the same study showed survivability at 20 °C and 50%RH was significantly less (4–5 days), further suggesting the humidity may play a significant role in virus survival.
The persistence of virus on both paper and polymer currency is of particular significance, considering the frequency of circulation and the potential for transfer of viable virus both between individuals and geographic locations. While other studies have shown that paper notes harbour more pathogens than polymer notes [38], this data demonstrates that SARS-CoV-2 persists on both paper notes and polymer notes to at least 28 days at 20 °C, albeit with a faster rate of inactivation on polymer notes. Data presented in this study for banknotes is significantly longer than reported for other respiratory viruses such as Influenza A (H3N2) which demonstrated survival up to 17 days at room temperature [39]. It is also noted that prior to SARS-Cov-2 being declared a pandemic, China had commenced decontamination of its paper-based currency, suggesting concerns over transmission via paper banknotes existed at the time [40, 41]. The United States and South Korea have also quarantined banknotes as a result of the pandemic [42, 43]. It is important to note that after 28 days, infectious SARS-CoV-2 was also recovered from stainless steel, vinyl and glass, suggesting survivability on paper or polymer banknotes was not very different from the other non-porous surfaces studied.
The persistence on glass is an important finding, given that touchscreen devices such as mobile phones, bank ATMs, supermarket self-serve checkouts and airport check-in kiosks are high touch surfaces that may not be regularly cleaned and therefore pose a transmission risk of SARS-CoV-2. It has been demonstrated that mobile phones can harbour pathogens responsible for nosocomial transmission [44], and unlike hands, are not regularly cleaned [45]. The data presented in this study correlates well with previously published data for Influenza A (H1N1) which recovered infectious virus up to 22 days at 22 °C and 7 days at 35 °C [37]. The persistence of SARS-COV-2 on glass and vinyl (both common screen and screen protector materials, suggest that touchscreen devices may provide a potential source of transmission, and should regularly be disinfected especially in multi-user environments.
The persistence of both SARS and SARS-CoV-2 on cotton has been demonstrated to be significantly shorter than on non-porous surfaces [11, 25]. The data presented here also show a significant decrease in titre of the recovered virus after just 1 h drying at room temperature (20 °C) the amount of virus recovered from cotton swatches was approximately 99% less than for comparable virus recovery time points for non-porous material. To verify the reduced recovery on cotton, the virus was eluted 5 min after depositing on the cotton, as well as 1 h, the titre of the recovered virus after 5 min was similar to that of non-porous surfaces (data not shown) suggesting the process of drying down was a significant factor for cotton material but not from the non-porous surfaces. Recovery of virus from porous substrates is also likely to be reduced compared to non-porous substrates due to adherence of the virus to the fabric fibres. When the rate of viral inactivation is considered over time rather than the gross reduction from the initial inoculum there is a more subtle difference from the non-porous surfaces. The D values for cotton at 20 °C, when compared to other materials, are not significantly different from other substrates (eg. 5.6 days for cotton vs. 6.3 days for vinyl), and the slopes of the line which suggests the decay rate of the virus is similar across substrates. This study also demonstrates significantly longer survival times on cotton (7 days) than previous reported [11, 25]. This difference could be due to differences in the types of cotton material used, the current study used 100% cotton cloth, while previous studies used either a cotton gown or cotton t-shirt.
Conclusions
Availability of data and materials
Abbreviations
ASTM: American Society for Testing and Materials
ATM: Automatic teller machine
BSCII: Biological Safety Cabinet, Class 2
BSA: Bovine serum albumin
CO2 : Carbon dioxide
CT: Cycle threshold
DMEM: Dulbecco’s Modified Eagle Medium
E gene: Envelope gene of SARS-CoV-2
N gene:Nucleocapsid gene of SARS-CoV-2
N95: Non-oil 95 mask
PBS: Phosphate buffered saline
RH: Relative humidity
RT-PCR: Reverse transcription polymerase chain reaction
RdRp: Ribonucleic acid dependant ribonucleic acid polymerase
SARS: Severe Acute Respiratory Syndrome
SARS-CoV-2:Severe Acute Respiratory Syndrome Coronavirus 2
TCID50: Tissue Culture Infectious Dose—Fifty
U/V: Ultraviolet
WHO: World Health Organisation
V/V: Volume per volume
References
- Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci U S A. 2020;117(22):11875–7.
CAS Article - Morawska L, Milton DK. It is time to address airborne transmission of COVID-19. Clin Infect Dis. 2020.
PubMed PubMed Central - Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci. 2020;117(26):202009637.
- van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–7. https://doi.org/10.1056/NEJMc2004973
Article PubMed - Smither SJ, Eastaugh LS, Findlay JS, Lever MS. Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerg Microbes Infect. 2020;9(1):1415–7. https://doi.org/10.1080/22221751.2020.1777906
CAS Article PubMed PubMed Central - Cai J, Sun W, Huang J, Gamber M, Wu J, He G. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg Infect Dis. 2020;26(6):1343–5.
CAS Article - Julian TR, Leckie JO, Boehm AB. Virus transfer between fingerpads and fomites. J Appl Microbiol. 2010;109(6):1868–74.
CAS Article - Rolfe T, Nitti M. Touchscreens: the mosquito of the digital age. 2016. https://emist.com/infection-prevention-touchscreens-are-contaminated/
- Rusin P, Maxwell S, Gerba C. Comparative surface-to-hand and fingertip-to-mouth transfer efficiency of gram-positive bacteria, gram-negative bacteria, and phage. J Appl Microbiol. 2002;93(4):585–92.
CAS - Kasloff SB, Strong JE, Funk D, Cutts TA. Stability of SARS-CoV-2 on critical personal protective equipment. medRxiv. 2020;2020.06.11.20128884.
- Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer. Beitrag zur Kollekt Behandlung pharmakologischer Reihenversuche. 1931;7:1–4.
- Spearman C. The method of “right and wrong cases” (‘constant stimuli’) without Gauss’s formulae. Br J Psychol 1908–1920. 1908;2(3):227–42.
- Sattar SA, Springthorpe VS, Adegbunrin O, Zafer AA, Busa M. A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. J Virol Methods. 2003;112(1–2):3–12.
CAS Article - ASTM E2197. Standard quantitative disk carrier test method for determining bactericidal, virucidal, fungicidal, mycobactericidal, and sporicidal activities of chemicals. ASTM Int. 2015. https://www.astm.org/Standards/E2197.htm
- Kim Y, Yang M, Goyal SM, Cheeran MCJ, Torremorell M. Evaluation of biosecurity measures to prevent indirect transmission of porcine epidemic diarrhea virus. BMC Vet Res. 2017;13(1):1–9.
- Lee SS, Wong NS. Probable transmission chains of Middle East respiratory syndrome coronavirus and the multiple generations of secondary infection in South Korea. Int J Infect Dis. 2015;38:65–7. https://doi.org/10.1016/j.ijid.2015.07.014
Article PubMed PubMed Central - Sizun J, Yu MWN, Talbot PJ. Survival of human coronaviruses 229E and OC43 in suspension and after drying on surfaces: A possible source of hospital-acquired infections. J Hosp Infect. 2000;46(1):55–60.
CAS Article - La Scola B, Le Bideau M, Andreani J, Hoang VT, Grimaldier C, Colson P, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39(6):1059–61.
Article - Kam K, Yung CF, Cui L, Tzer Pin Lin R, Mak TM, Maiwald M, et al. A well infant with coronavirus disease 2019 with high viral load. Clin Infect Dis. 2020;71(15):847–9.
CAS Article - Huang Y, Chen S, Yang Z, Guan W, Liu D, Lin Z, et al. SARS-CoV-2 viral load in clinical samples from critically Ill patients. Am J Respir Crit Care Med. 2020;201(11):1435–8.
CAS Article - Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–9. https://doi.org/10.1056/NEJMc2001737
Article PubMed PubMed Central - Chan KH, Peiris JSM, Lam SY, Poon LLM, Yuen KY, Seto WH. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol. 2011. https://doi.org/10.1155/2011/734690
Article PubMed PubMed Central - Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen H-L, Chan MCW, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10.
Article - Lai MYY, Cheng PKC, Lim WWL. Survival of severe acute respiratory syndrome coronavirus. Clin Infect Dis. 2005;41(7):e67-71.
CAS Article - Aboubakr HA, Sharafeldin TA, Goyal SM. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound Emerg Dis. 2020.
PubMed PubMed Central - Biryukov J, Boydston JA, Dunning RA, Yeager JJ, Wood S, Reese AL, et al. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. mSphere. 2020;5(4):1–9.
Article - Casanova LM, Jeon S, Rutala WA, Weber DJ, Sobsey MD. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol. 2010;76(9):2712–7.
CAS Article - van Doremalen N, Bushmaker T, Munster V. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Eurosurveillance. 2013;18(38):20590.
Article - Ratnesar-Shumate S, Williams G, Green B, Krause M, Holland B, Wood S, et al. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J Infect Dis. 2020;222(2):214–22.
CAS Article - Schuit M, Ratnesar-Shumate S, Yolitz J, Williams G, Weaver W, Green B, et al. Airborne SARS-CoV-2 is rapidly inactivated by simulated sunlight. J Infect Dis. 2020;222(4):564–71.
CAS Article - Guillier L, Martin-Latil S, Chaix E, Thébault A, Pavio N, Le Poder S, et al. Modelling the inactivation of viruses from the Coronaviridae family in response to temperature and relative humidity in suspensions or surfaces. Appl Environ Microbiol. 2020;80(21):6807–18. https://doi.org/10.1128/AEM.01244-20.
Article - Fisher D, Reilly A, Kang A, Zheng E, Cook AR, Anderson DE. Seeding of outbreaks of COVID-19 by contaminated fresh and frozen food. bioRxiv. 2020.
- Fischer R, Judson S, Miazgowicz K, Bushmaker T, Prescott J, Munster VJ. Ebola virus stability on surfaces and in fluids in simulated outbreak environments. Emerg Infect Dis. 2015;21(7):1243–6.
CAS Article - Mbithi JN, Springthorpe VS, Sattar SA. Effect of relative humidity and air temperature on survival of hepatitis A virus on environmental surfaces. Appl Environ Microbiol. 1991;57(5):1394–9.
CAS Article - Warnes SL, Little ZR, Keevil CW. Human coronavirus 229E remains infectious on common touch surface materials. MBio. 2015;6(6):1–10.
Article - Dublineau A, Batéjat C, Pinon A, Burguière AM, Leclercq I, Manuguerra JC. Persistence of the 2009 pandemic influenza a (H1N1) virus in water and on non-porous surface. PLoS ONE. 2011;6(11):e28043.
- Vriesekoop F, Russell C, Alvarez-Mayorga B, Aidoo K, Yuan Q, Scannell A, et al. Dirty money: an investigation into the hygiene status of some of the world’s currencies as obtained from food outlets. Foodborne Pathog Dis. 2010;7(12):1497–502.
- Thomas Y, Vogel G, Wunderli W, Suter P, Witschi M, Koch D, et al. Survival of influenza virus on banknotes. Appl Environ Microbiol. 2008;74(10):3002–7.
- Yeung J. China is disinfecting and destroying cash to contain the coronavirus. 2020. https://edition.cnn.com/2020/02/17/asia/china-is-disinfecting-cash-coronavirus-intl-hnk-scli/index.html.
- Wibawa T. China cleans bank notes in bid to limit coronavirus COVID-19 spread. ABC news (Australia). 2020. https://www.abc.net.au/news/2020-02-21/china-cleaning-money-limit-coronavirus-covid-19/11983364.
- Schroeder P, Irrera A. Fed quarantines U.S. dollars repatriated from Asia on coronavirus caution. 2020. https://www.reuters.com/article/us-health-coronavirus-fed-dollars/fed-quarantines-us-dollars-repatriated-from-asia-on-coronavirus-caution-idUSKBN20T1YT.
- Choi H. S.Korea’s central bank burns, quarantines cash in coronavirus precaution. 2020. https://uk.reuters.com/article/health-coronavirus-southkorea-money/s-koreas-central-bank-burns-quarantines-cash-in-coronavirus-precaution-idUKL4N2AZ1TL
- Brady RRW, Wasson A, Stirling I, McAllister C, Damani NN. Is your phone bugged? The incidence of bacteria known to cause nosocomial infection on healthcare workers’ mobile phones. J Hosp Infect. 2006;62(1):123–5. CAS Article
- 45.Olsen M, Campos M, Lohning A, Jones P, Legget J, Bannach-Brown A, et al. Mobile phones represent a pathway for microbial transmission: a scoping review. Travel Med Infect Dis. 2020;35(April):101704. https://doi.org/10.1016/j.tmaid.2020.101704.
Article PubMed PubMed Centr
